Joshua Hatterschide of Carolyn Coyne's Lab was awarded a prestigious F32 grant from the National Institute of Environmental Health Sciences (NIEHS). His research proposal, entitled, "Defining cell-intrinsic trophoblast defenses to HCMV infection," won Joshua a two-year award to continue his research in Allergy and Infectious Diseases.
Tell us about your academic background
I attended The Ohio State University as an undergraduate, where I majored in Biochemistry. I then received my PhD from the University of Pennsylvania in Cell and Molecular Biology in 2022.
Describe your work
I have always had a fascination with modeling complex cellular environments in vitro. In my graduate work, I studied molecular mechanisms that human papillomaviruses (HPVs) use to manipulate epithelial differentiation. In many of these experiments, I employed organotypic squamous epithelial cultures, which are air-liquid interface cell culture systems that model the stratification and differentiation of the skin or mucosal epithelia.
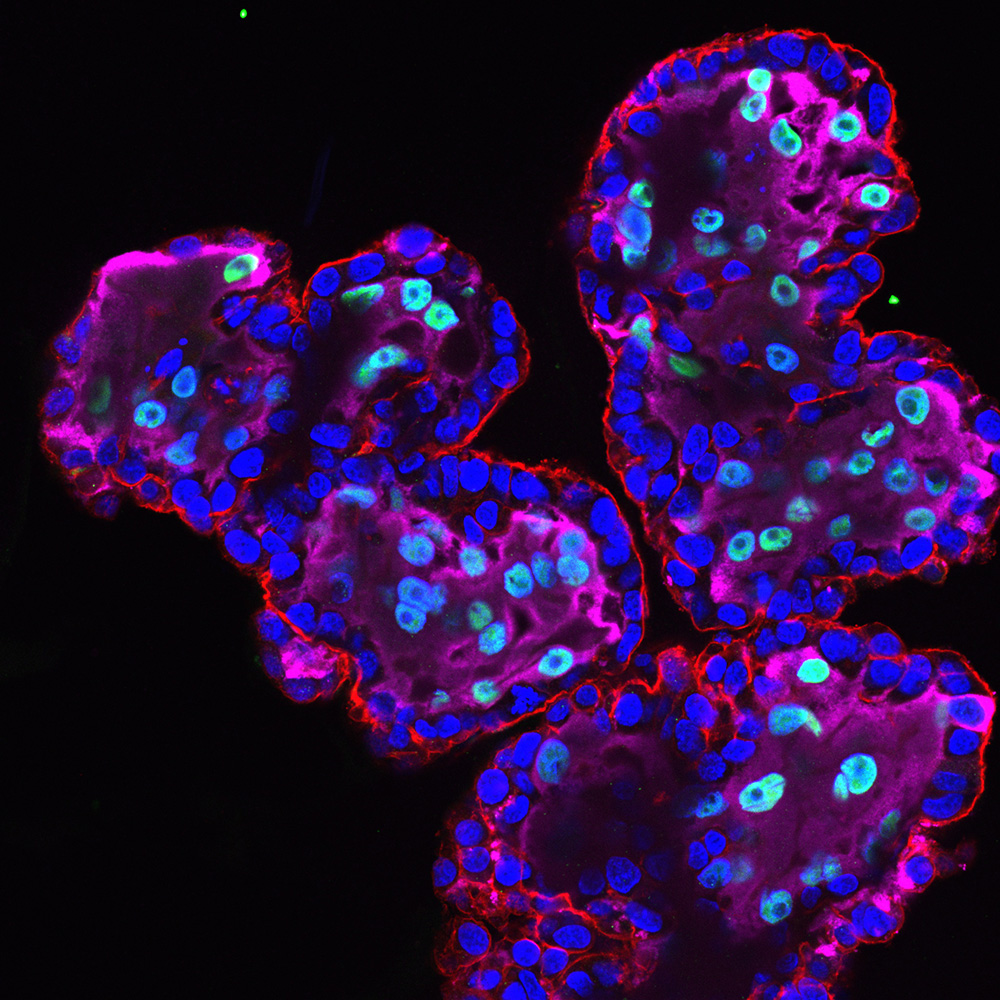
Just before I joined Dr. Carolyn Coyne’s lab at Duke, they had published the isolation of several types of organoids from full-term placental tissue, including fetal-derived trophoblast organoids and maternal-derived decidua epithelial organoids. Organoids are three-dimensional cell culture systems that share many advantages with organotypic cultures including differentiation into many of the cell types found in their corresponding tissues. However, organoids have the added benefit of being continuously propagatable and more easily genetically engineered for mechanistic research. I joined the Coyne lab to study infections at the maternal-fetal interface and learn to culture placenta-derived organoids.
What you plan to do with the award
Placental trophoblasts are the primary barrier between maternal and fetal circulation. Thus, vertically transmitted viruses must bypass trophoblasts to infect the developing fetus. In my NRSA F32, I proposed to use trophoblast organoids to investigate how these cells respond to and defend against one of the most commonly vertically-transmitted pathogens: human cytomegalovirus (HCMV). I have preliminarily determined a putative cellular defense program that dominates the response of trophoblasts to HCMV infection and may contribute to restricting the virus. The two aims of my grant are 1) to test whether this program restricts HCMV replication in trophoblasts, and 2) to identify whether this program has unintended consequences that impair the normal function of trophoblasts. Projects such as this will enhance our understanding of how viruses overcome placental defenses and ultimately cause congenital disease.